Background
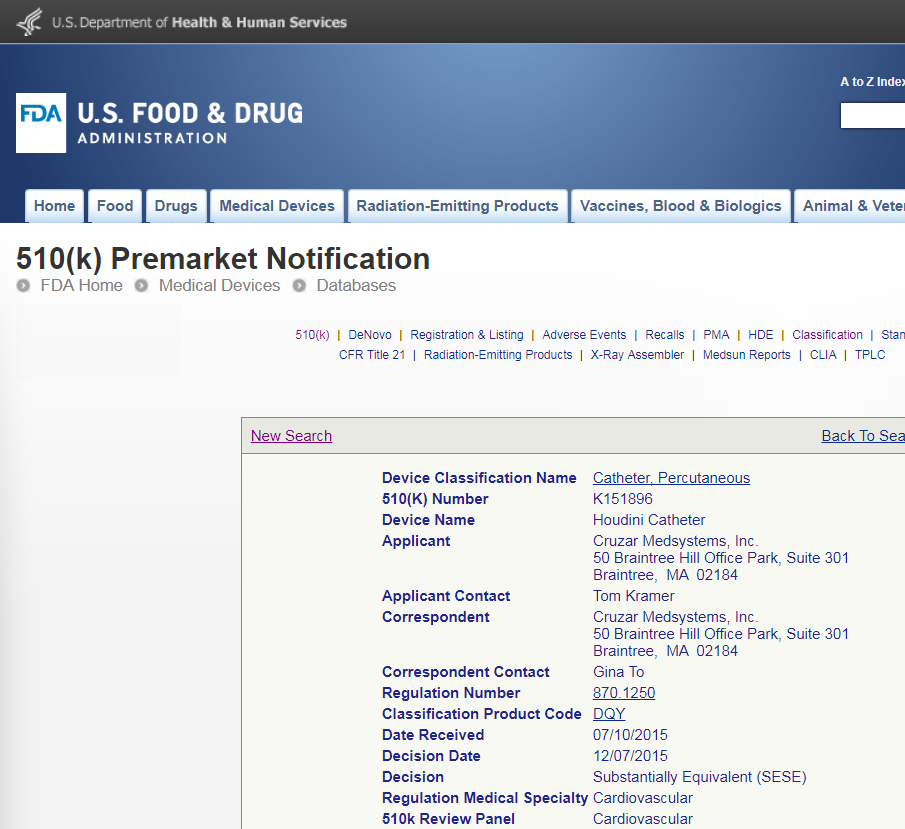
The Houdini Catheter by Cruzar Medsystems is a balloon catheter for guidewire placement in the most difficult to cross vascular obstructions. Sirius Engineering partnered with Cruzar Medsystems from early incubation and development to obtain a 510(k) Premarket Notification approval for the device.
We have set up the Quality System for Cruzar, which is compliant to the FDA QSR requirements. This includes authoring the quality manual, Standard Operating Procedures (SOPs), and forms. The quality system is maintained by Sirius through updates that come from new and revised international standards, internal and external audits, and industry and customer feedback.
With clinical and surgeon feedback, Sirius advanced the technology and released the second generation of the device, the Houdini Cross Support Catheter. Sirius Engineering serves as the manufacturer and distribution warehouse for both products in the Houdini Device Family.
How the Magic of the Houdini works
Development at a glance
Proof of concept for the mechanical actuation and inflation of the the dual lumen catheter
Verification and Validation testing of anchoring and traversing through the vasculature to simulated lesions
Design of the packaging for optimal physician and nurse experience
FDA approval of the 510(k) submission and market release of the Houdini