The Sirius Approach
Sirius is committed to developing practical solutions that improve patient outcomes and enhance healthcare delivery. With a dedicated focus on process excellence, we design, refine, and manufacture medical devices that meet the highest standards of quality and safety. Our proven development process ensures that each product undergoes rigorous testing and validation, resulting in a product that is reliable and can be manufactured repeatedly with high surgeon satisfaction.
Phase 1: Proof of concept
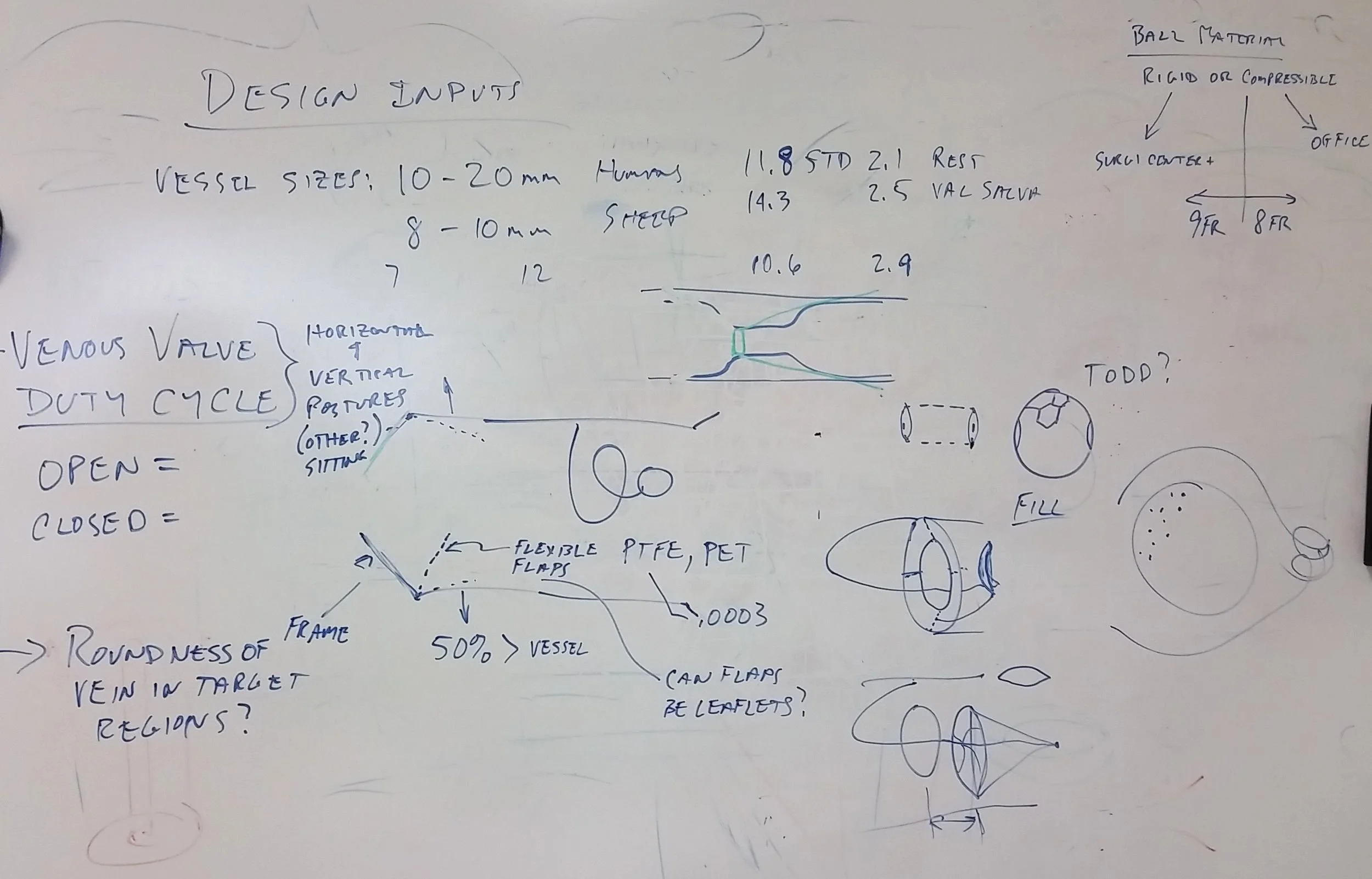
Consider Sirius to kick-off your Design Control process. You will join in our brainstorming sessions to create early prototypes for your ideas. Rapid iterations with multiple prototype models challenge the design inputs to realize and to demonstrate proof of your concept.
Phase 2: feasibility
Following the proof of concept stage, Sirius Engineering refines the design, ensuring functionality and suitability for manufacturing. This involves selecting appropriate materials and suppliers, as well as initiating fabrication of your GMP assembly line.
Phase 3: Qualification
Pilot production is then initiated in our ISO-7 cleanroom, and comprehensive testing is conducted on your production level product in a simulated environment. We verify and validate your design inputs with documented evidence that ensures FDA compliance. We accompany you all the way through the FDA submission process to expedite your approval.
Phase 4: Design transfer
Initial inventory is readied for the market launch of your product. Improvements to the manufacturing process are made to reduce costs and boost manufacturing efficiency. From there, manufacturing efforts continue, focusing on meeting demand for the current device while also exploring advancements for future generations of the medical device.