Background
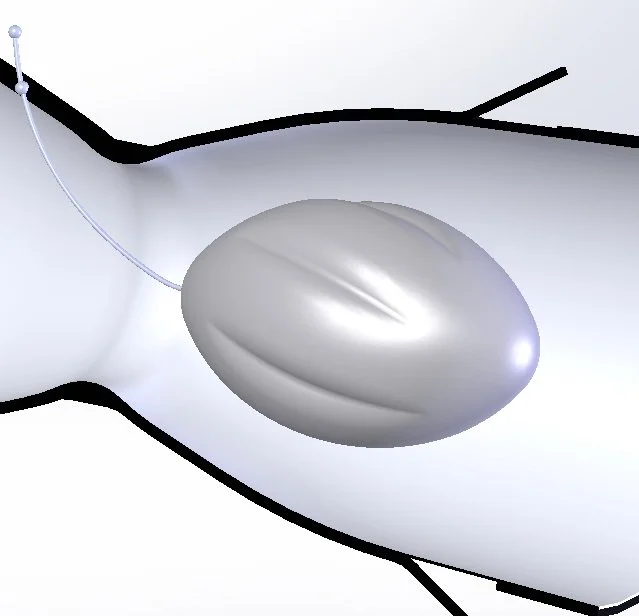
The Innovein, Inc. Elevate Valve was developed to treat Chronic Venous Insufficiency (CVI), a leg vein condition that causes symptoms like varicose veins, pain when standing up, swelling, and ulcers. Normally, valves in your leg veins keep blood flowing back up to your heart, but with CVI, damage to the leg vein valves has reached a point where blood pools in your legs. The Elevate Valve prosthetic implant solves this problem by adding new valves to assist the damaged or failing natural valves.
Sirius partnered with Dr. Al Chin and Dr. Austin Walker to conceive, incubate and develop the vein valve solution which led to the formation of InnoVein, Inc. This development utilized Sirius’ expertise in rapid prototype development, Nitinol shape-setting and membrane coating technology resulting in a proprietary replacement valve device. After Proof of Concept and Feasibility phases of development, the vein valve was taken into clinical trials in Australia.
Subsequently, Innovein was spun out into a stand-alone company, while Sirius continued to provide implant design R&D, shipping and packaging testing and designs, the FDA QSR compliant Quality System, and Quality Assurance.
Development at a glance
Developing and manufacturing a PTFE Coated Nitinol Valve using precision fluidized heat baths and a proprietary coating method.
Designing and manufacturing a delivery system capable of discrete positioning and accurate deployment.
Simulated Computer modeling and flow simulations were used to identify optimal placement and geometry of key design features
Manufacturing, testing, and inspection of numerous critical tolerances and processes to yield only devices fit for use.